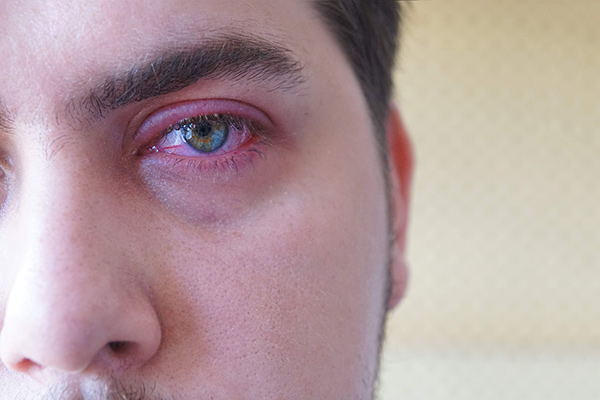
Novel Therapeutics for Ocular Surface Disease
Aperta Biosciences is an emerging biopharma company focused on the development of novel treatments for Ocular Surface Disease (OSD), including blepharitis, ocular rosacea, meibomian gland dysfunction, and dry eye disease.
Our Team
Founded and led by an executive, operational, and regulatory team with decades of experience in ophthalmic indications and in the development of both novel and repurposed therapeutics.